Abstract
Background: There is no current standard of care for relapsed/refractory mantle cell lymphoma (MCL). Current recommendations include a number of second-line treatment options including chemotherapy regimens, such as bendamustine and rituximab (BR), or agents targeting signaling pathways including bortezomib, lenalidomide, and ibrutinib. We conducted a multi-center review of outcomes for patients with relapsed/refractory MCL to identify trends in therapy and response to treatment.
Methods: The primary objective of this study was to compare progression free survival (PFS) of first salvage therapies utilized in MCL, defined as time from first relapse to death/progression. Secondary objectives include assessing differences in response rates, duration of response, and overall survival (OS). We included patients ≥18 years old with relapsed/refractory MCL treated between January 1, 2002 and August 31, 2016. Patients were excluded if they had not relapsed following initial treatment, had insufficient data to evaluate, or received an alternative salvage therapy to the agents we were evaluating. Agents of interest included bendamustine and rituximab (BR), bortezomib, lenalidomide, and ibrutinib. Descriptive statistics were used to summarize patient demographic information. Cox proportional hazards models and log rank tests were used to determine differences in progression-free survival, and survival curves were generated using the Kaplan-Meier method.
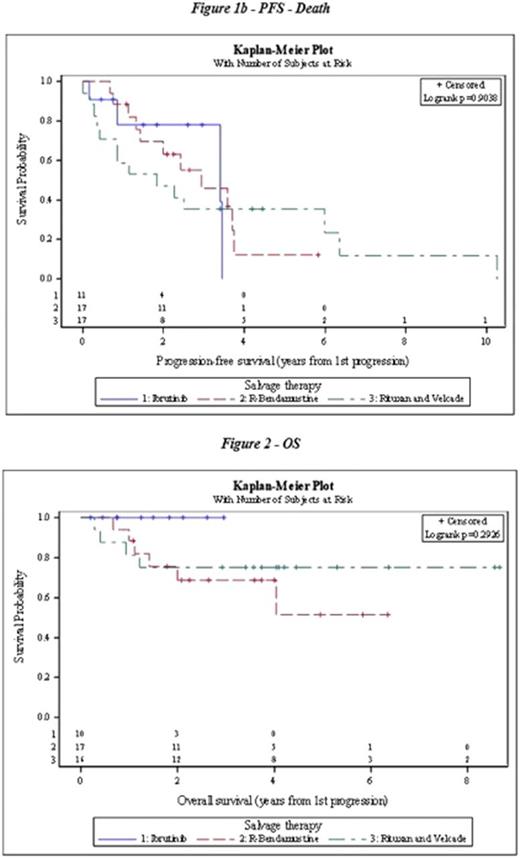
Results: Of the 484 patients evaluated, 50 patients met inclusion criteria for the study. Median age at diagnosis was 64 years old and the majority of patients were male (80%). Twenty-two patients (46%) received autologous transplant in first remission. Patients received bendamustine-rituximab (n=20), bortezomib +/-rituximab (n=18), or ibrutinib (n=12). ORR was 65% for BR, 50% for bortezomib, and 67% for ibrutinib, and CR rates were 60%, 50%, and 42% respectively. No significant difference was found in the response rates of these agents (p= 0.239).The median progression-free survival for bendamustine-rituximab was 2.9 years, for bortezomib +/- rituximab was 1.8 years, and for ibrutinib was 3.4 years (figure 1). There was no significant difference between these therapies (p=0.90). The overall survival at 2 years was 100% for ibrutinib, 75.6% for BR, and 75% for bortezomib +/-rituximab (figure 2).
Conclusions: There was no significant difference in response rate, median PFS, or OS when comparing salvage treatment with bendamustine and rituximab, bortezomib +/-rituximab, or ibrutinib as first salvage approach in relapsed/refractory MCL. Larger studies are needed to better define the appropriate sequence of therapies in MCL, and clinical decisions should incorporate patient-specific factors and preferences given similar outcomes seen across salvage approaches.
Park: Seattle Genetics: Research Funding; Takeda: Research Funding; Gilead: Speakers Bureau; Teva: Consultancy, Research Funding; Cornerstone Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Fenske: Celgene: Consultancy; Pharmacyclics: Consultancy; Sanofi: Consultancy. Hamadani: Celgene, Cellerant, Jansen, MedImmune: Consultancy; Sanofi Genzyme: Research Funding, Speakers Bureau; Takeda, Otsuka, MedImmune, Merck, ADC Therapeutics: Research Funding. Flowers: V Foundation: Research Funding; Prime Oncology: Research Funding; Educational Concepts: Research Funding; Genentech/Roche: Consultancy, Research Funding; Research to Practice: Research Funding; Acerta: Research Funding; Burroughs Welcome Fund: Research Funding; Janssen Pharmaceutical: Research Funding; OptumRx: Consultancy; Spectrum: Consultancy; Millennium/Takeda: Research Funding; National Cancer Institute: Research Funding; Abbvie: Consultancy, Research Funding; Onyx: Research Funding; TG Therapeutics: Research Funding; Gilead: Consultancy; Bayer: Consultancy; Celgene: Consultancy, Research Funding; National Institutes Of Health: Research Funding; Seattle Genetics: Consultancy; Infinity: Research Funding; Clinical Care Options: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding. Cohen: Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; LAM Therapeutics, Inc: Research Funding; Takada: Research Funding; Bristol Myers Squibb: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioinvent: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal